Antifreeze is a crucial automotive additive, preventing your engine coolant from freezing. It is especially important over winter time. If antifreeze is low or absent, your car's performance will be impacted.
Antifreeze helps ensure the water used to cool your engine does not freeze; but it also helps prevent it from boiling in summer months.
The water pump directs this water around your engine. A component called ethylene glycol is key to antifreeze; it inhibits the ability of water molecules to expand and crystallize in low temperatures - essentially lowering the freezing point.
Without antifreeze, water of freezes at 0 degrees Celsius. But with antifreeze (50/50 water and ethylene glycol) the freezing point falls to -37 degrees. The boiling point rises slightly to 106 degrees.
Each year, before the winter set in, open your bonnet and top up your antifreeze as required.
Here we answer common questions about antifreeze…
Antifreeze vs coolant: What’s the difference?
Once antifreeze is diluted with distilled or deionised water (at a 50:50 ratio), it is known as coolant. These terms are often used synonymously.
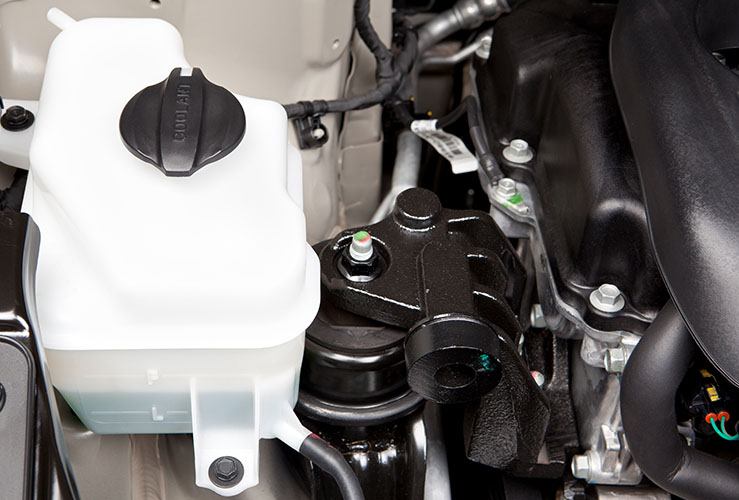
What is a coolant reservoir tank?
Every petrol or diesel engine needs a coolant reservoir tank. You’ll find it by opening the bonnet and looking near the engine.
The reservoir holds coolant which is used to cool the engine.
Without sufficient coolant, the engine may overheat or seize up.
Where is the coolant reservoir located?
The coolant reservoir is where you should add antifreeze for your car engine. You'll find the coolant reservoir with warning stickers on the lid.
If you’re unsure where to pour antifreeze in your car, your vehicle handbook will show you where it is and how to do it.
The reservoir should be transparent enough to see how much coolant is inside.
Naturally, if there is a sufficient amount (up to the ‘full’ line), you do not need to open it. If a top up is necessary, unscrew the coolant cap and pour in the antifreeze to the ‘maximum’ mark.
Should I open the coolant reservoir when it’s hot?
For safety reasons, ensure the engine is cool before you touch the coolant reservoir cap (or any part of the engine).
Wait at least 30 minutes from the last time it was used.
How to check antifreeze in my car?
After locating the coolant reservoir, check the coolant level is between the ranges indicated. If it is too low, you’ll need to top it up.
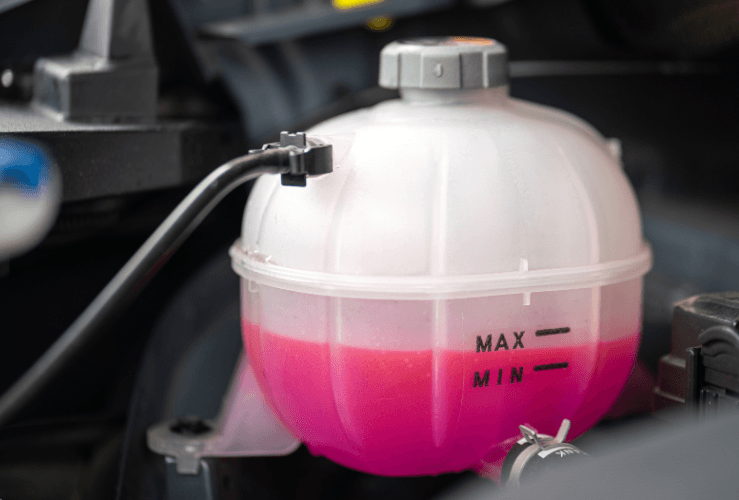
How to add the antifreeze mix?
Here’s how to add antifreeze to a car: Look for the coolant reservoir - it will likely be a semi-transparent whitish shade. Carefully unscrew the lid a little and release any build of pressure. Then remove the lid fully and add the antifreeze up to the ‘fill’ line.
Best antifreeze for your car?
A wide choice of antifreeze products are available to buy, but you should always use the type of antifreeze recommended by the manufacturer.
You should be able to find this information in your handbook.

Is it OK to add water to the coolant reservoir?
If the product is concentrated antifreeze, you’ll need to dilute it with distilled water at a ratio of 50:50.
The water must be distilled so it is free of any minerals that build up in the radiator and reduce flow. Do not use regular tap water. You might also use deionized water.
Do you add coolant to the radiator or reservoir?
You must add coolant to the coolant reservoir - not the radiator.
As mentioned, if you buy concentrated antifreeze, you’ll need to dilute it with distilled water at a 50:50 ratio. However, carefully read the instructions on the bottle.
How often should I add antifreeze to my car?
If your antifreeze/coolant drops below the range indicated on the coolant reservoir you should top it up.
Your handbook will explain how often you should expect to top it up (usually a minimum of 30,000 miles; or 150,000 miles if using long life - see Types of Antifreeze section below).
Older cars may need more regular top-ups.
Cost to replace antifreeze?
Antifreeze replacement costs depend on how you do it.
Buying antifreeze and replacing it yourself will cost around £40. Getting a mechanic to do it for you could cost around £70.
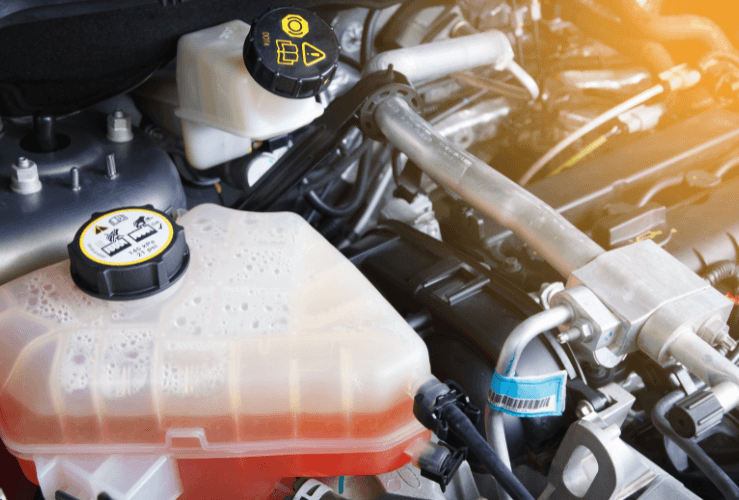
Which antifreeze colour for my car?
Antifreeze colour coding explained
Check your car manual to find out which antifreeze you need. In short, you’ll probably need either red or orange antifreeze. However, as mentioned below, antifreeze colour-coding is not always uniform.
You're unlikely to come across green antifreeze, as this was made to an old formula that uses Inorganic Additive Technology (IAT). It was used into the 1990s.
- Orange antifreeze signifies the use of neutralised organic acids, which helps inhibit corrosion. It may also be red.
- Red antifreeze signals Hybrid Organic Acid, which combines the benefits of green and orange antifreeze. Confusingly, it may also be orange!
Can I mix different antifreeze for my car?
No. Mixing different antifreeze types could impair performance and increase corrosion.
Refer to your vehicle handbook for the correct type of antifreeze to use.
What happens if you put too much antifreeze in your car?
If you put too much antifreeze/coolant in your car, it will likely be released from an overflow hose - probably resulting in a puddle under your car.
However, excessive coolant overflow could damage electrical systems if they come into contact with wiring.
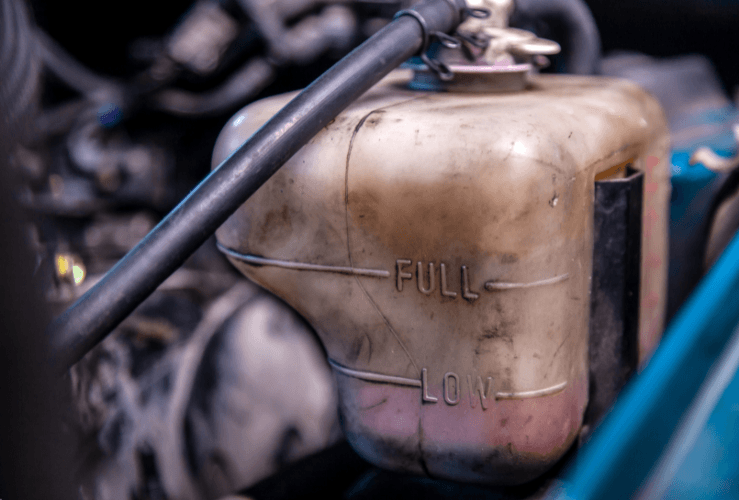
Can I drive with a cracked coolant reservoir?
A cracked coolant reservoir should be fixed promptly, since it may be causing coolant to leak. However, it’s likely you will be able to drive for some time with a cracked coolant reservoir - depending on the extent of the damage.
While a crack may not be visible to the naked eye, if your coolant is running low sooner than expected, you’re experiencing engine overheating, or see visible leaks, it suggests your coolant reservoir is damaged.
Bottom line: get it fixed as soon as you can.
Can I drive with an empty coolant reservoir?
You should not drive with an empty coolant reservoir. Coolant is essential for drawing heat away from your engine.
Without sufficient levels of coolant, your engine could overheat or seize up - potentially resulting in permanent damage (e.g., pistons welding to cylinders) that will be expensive to repair. In some cases, repairs may be impossible or uneconomic (i.e. it may be cheaper to buy a new vehicle).
Why does my coolant reservoir keep emptying?
If your coolant keeps emptying soon after being refilled, there could be a number of causes. The most likely are: a leak in the radiator, heater core, or a hose; the radiator cap is jammed open; a leak in the water pump/water pump seal; or a blown head gasket, releasing coolant into the combustion chamber and exiting through the exhaust.
Why does it smell like antifreeze inside my car?
If you notice the smell of antifreeze in your vehicle, it’s a sign there’s a coolant leak - causing the distinct antifreeze smell to be pumped out through your air vents.
It’s important to get any coolant leak repaired promptly.
How to fix a coolant reservoir leak?
You may be able to fix a small crack in your coolant reservoir with epoxy resin or purpose-made sealant. However, if the damage is extensive, replacement is the best course of action.
Can a bad thermostat cause bubbling in the coolant reservoir?
Yes. When a thermostat fails, it doesn’t open and close when it needs to.
With the resultant unregulated airflow, a bubbling effect may occur inside the coolant reservoir or the radiator.
Symptoms of low antifreeze
Here are the most common signs that your antifreeze levels are too low:
- Engine overheating: Without sufficient antifreeze, your vehicle’s engine is likely to overheat. If this does happen, you’ll see the engine’s temperature gauge rise. A dashboard warning light may come on (thermometer icon).
- Heater not working: If your vehicle’s heater starts to blow cool air, it’s a sign there’s coolant circulating through your heater core.
- Sweet smell: If you notice a sweet, syrupy smell inside and/or outside your car, it’s a sign you could have a coolant leak. By extension, your coolant levels will be low.
- Steam/vapour from the bonnet: If you see steam or vapour issuing from under your bonnet, this could also be a sign of a coolant leak.
- Coolant light or other warning: Some vehicles have dedicated coolant warning lights. A coolant leak is one of the most common triggers.
- Reduced engine performance: Without sufficient coolant, your vehicle may deliver reduced performance, or even go into ‘limp mode’ if it has one.
- Coolant puddle under car: If you see a patch of coolant underneath your vehicle, it's safe to assume your antifreeze is running low.
Safety precautions for handling antifreeze
Care should be taken when handling antifreeze, since it is toxic to both humans and animals.
Protective gear
- Wear chemical-resistant gloves whenever handling antifreeze.
- Use goggles to protect your eyes from any splashback.
- Wear a long sleeved shirt/long trousers to reduce skin exposure.
Avoid inhalation/ingestion
Antifreeze contains either ethylene glycol or propylene glycol, both of which are highly toxic to humans and animals.
Whenever handling antifreeze, ensure the area is very well ventilated.
Store antifreeze away from children and pets
Antifreeze has a sweet smell and taste, which can attract children and animals. As such, ensure antifreeze is clearly labelled and stored somewhere children and animals won’t come across it.
Wait until the engine is cool before accessing coolant
Do not open the radiator cap when the engine is still hot. If you do, you run the risk of serious burns, since the coolant will be highly pressurised and could spray out.
Deal with any spillages straight away
Use kitchen towels or kitty litter to soak up any spillages.
Get rid of any waste responsibly; do not pour it down the drain/sink, or leave it on the ground.
Dispose of old antifreeze responsibly
Never pour antifreeze down the drain, into toilets, or leave it when it can seep into the ground.
Instead, find a recycling centre or garage equipped to dispose of such hazardous material.
More on the environmentally safe disposal of antifreeze
As mentioned, antifreeze is toxic to humans and animals. It can also kill plants and contaminate soil. Additionally, it can leach into the groundwater, further threatening animal and plant life.
It’s no surprise, then, that antifreeze is considered hazardous under the Environmental Protection Act 1990 and the Hazardous Waste Regulations 2005. As such, if you pour it down the drain or toilet, or otherwise let it enter the environment, you would be breaking the law. Penalties include fines, or even criminal charges for repeat offences.
With the above in mind, it's important to find a safe way of disposing of antifreeze.
You can use Gov.uk’s tool for finding hazardous waste disposal services. Simply enter your postcode. In most cases, your local council will offer suitable services.
In London, contact the City of London’s hazardous waste collection service. It offers its collection service across London’s boroughs - except Hillingdon.
Additionally, some garages and MOT centres offer antifreeze disposal. However, these are not as common as oil or battery disposal services.
A note on antifreeze chemicals
Ethylene glycol is the most common main ingredient in antifreeze. It is also the most toxic to humans, animals and plants. Propylene glycol is sometimes used instead of ethylene glycol. The former is less toxic than the latter, but can still pose a serious risk to the environment.
Types of Antifreeze (Regular vs Long Life)
When it comes to antifreeze, it's not a case of "one size fits all." There are big formulation differences, and service intervals can vary.
Inorganic Additive Technology (IAT) (Green – "regular antifreeze")
- Green antifreeze nearly always uses "Inorganic Additive Technology (IAT)."
- It harnesses silicates and phosphates to help protect metal components from corrosion.
- It is considered a "regular antifreeze" – since it only lasts around 30,000 miles or 2 years.
- Green IAT antifreeze is used in older, pre-1990s vehicles, and is not suitable for modern engines – particularly those with aluminium blocks.
Organic Acid Technology (OAT) – "Long life antifreeze"
- OAT is used in modern mass-production vehicles like those from GM, Vauxhall, and Peugeot – i.e. those with aluminium parts.
- It is orange, red, or dark green.
- OAT is considered "long life antifreeze" because it lasts for about 150,000 miles or 5 years.
Hybrid Organic Acid Technology (HOAT) – Long life with extra protection
- HOAT is also considered "long life antifreeze" and uses organic acids + silicates to improve protection against corrosion.
- It is often yellow, turquoise, pink, or purple.
- HOAT should last 5 years or 150,000 miles.
- Do check your manual for more information, since multiple HOAT variants exist.
P-OAT (Phosphated OAT)
- P-OAT is designed for Asian-spec cooling systems, particularly in models made by Japanese and Korean marques.
- It is usually pink or blue.
- P-OAT is also considered a long life antifreeze.
A note on Regular vs Long Life Antifreeze
The majority of modern antifreeze products are considered 'long life' – lasting around 5 years or 150,000 miles. Only Inorganic Additive Technology (IAT) antifreeze is considered "regular" – lasting around 2 years or 30,000 miles.





